

Conversely on the interval a system with positive entropy has a closed invariant subset on which it is chaotic (although we won't prove this). Meanwhile on the circle $S^1$, it is known that every chaotic system has positive entropy, but that there exist chaotic systems with arbitrarily small entropy. Going the other way, on the space $ \Sigma_2$ of $(0,1)$-sequences, there exist chaotic systems with zero entropy. This means that on many spaces $X$, it is possible to construct non-chaotic systems with arbitrarily large topological entropy.
Meanwhile positive topological entropy is a local property, in the sense that if $f$ has positive entropy on a (possibly small) invariant set, then it has positive entropy on the entire space. if G 0 then the system is at the limit of reaction spontaneity. G -93000 - (T x -198) note that the enthalpy is given in kilojoules.
POSITIVE ENTROPY MEANS FREE
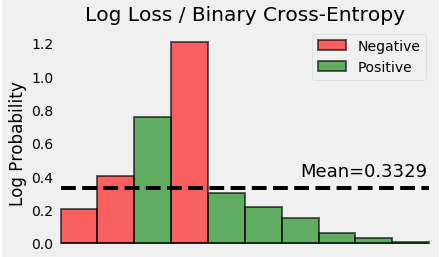
be positive according to the second law of thermodynamics: Ssystem eS +iS >0. The fact that both terms are negative means that the Gibbs free energy equation is balanced and temperature dependent: G H - TS. The extreme case, when the Entropy is highest happens when exactly half of the data belongs to each of the labels. information and is also defined as the negentropy NE: ISeq -S NE. a dataset with 45 Positive samples and 55 Negative samples has a very high Entropy. However in fact they are quite different: chaos is a global property, in the sense that it makes an assumption on the dynamics of $f$ across the entire space $X$. A high Entropy means the labels are in chaos. Furthermore, the system does not affect the entropy of its surroundings, since heat transfer between them does not occur. We can apply the second law of thermodynamics to chemical reactions by noting that the entropy of a system is a state function that is directly proportional to the disorder of the system. The entropy of various parts of the system may change, but the total change is zero. By definition, neither heat nor work can be transferred between an isolated system and its surroundings.
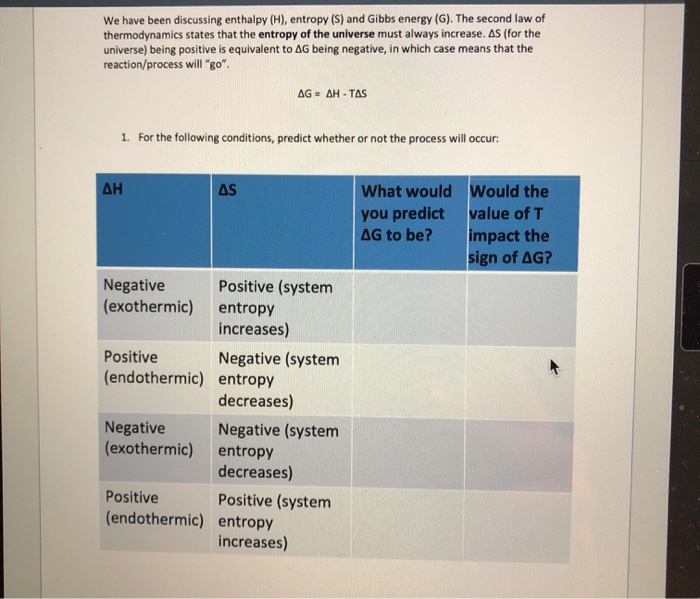
Naively one might infer that the two concepts are related. This result, which has general validity, means that the total change in entropy for a system in any reversible process is zero. Chaos and positive entropy are two different ways of measuring the amount of complexity (or instability) in a dynamical system.


 0 kommentar(er)
0 kommentar(er)
